 This is a fairly straightforward method of tracking a neutralisation reaction by monitoring the temperature.
This is a fairly straightforward method of tracking a neutralisation reaction by monitoring the temperature.
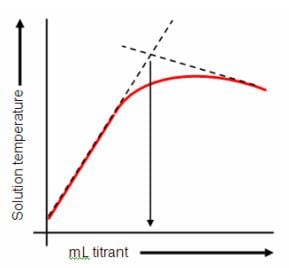
The neutralisation reaction between hydrochloric acid and sodium hydroxide is exothermic. So the highest temperature is found when enough acid has been added to neutralise all of the sodium hydroxide.
After this point the temperature drops because the acid now being added is at a lower temperature than the reaction mixture.



