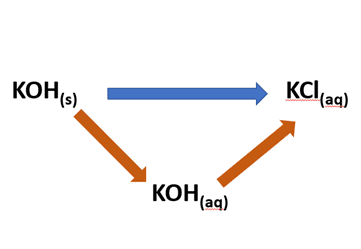
 Solid potassium hydroxide can be converted into potassium chloride solution by two different routes:
Solid potassium hydroxide can be converted into potassium chloride solution by two different routes:
A direct route whereby potassium chloride solution is made by adding solid potassium hydroxide directly to hydrochloric acid. and an indirect route which involves two steps. In the first of these solid potassium hydroxide is dissolved in water. The resulting potassium hydroxide solution is then added to hydrochloric acid to form potassium chloride solution:
According to Hess’s Law the overall enthalpy change involved in converting solid potassium hydroxide into potassium chloride solution will be the same no matter whether the direct or indirect route is taken.
The aim of this experiment is to confirm Hess’s Law.



